PVReg-Profiler
A comprehensive global PV requirement profiling and analysis system
BACKGROUND
Developed in partnership with ISoP, the Global Pharmacovigilance Professional Certification (GPPC) is the core project of the Institute of Pharmacovigilance (IPV). This certification is based upon series of multiple-choice questions (MCQs) selected to match the array of professional profiles elaborated by ISoP’s Special Interest Group on PV Professional Qualification Framework.
The content of the GPPC may need to be customised according to safety regulatory framework applicable in the regulatory system under the scope of the persons applying for certification. To collect, manage and characterise the PV-relevant requirements of a wide array country regulatory frameworks, IPV developed a Global Regulation Knowledge Management system named PVReg.
METHODS
The PVReg-Profiler is a web-application for capturing the PV-relevant regulatory requirements applicable in individual countries. It includes about 400 standardised questions organised according to 14 modules (left column of Fig. 1-3). The main challenge was to make it capable of capturing, with a sufficient degree of details, the considerable diversity of requirement across the world without inflating the number of questions.
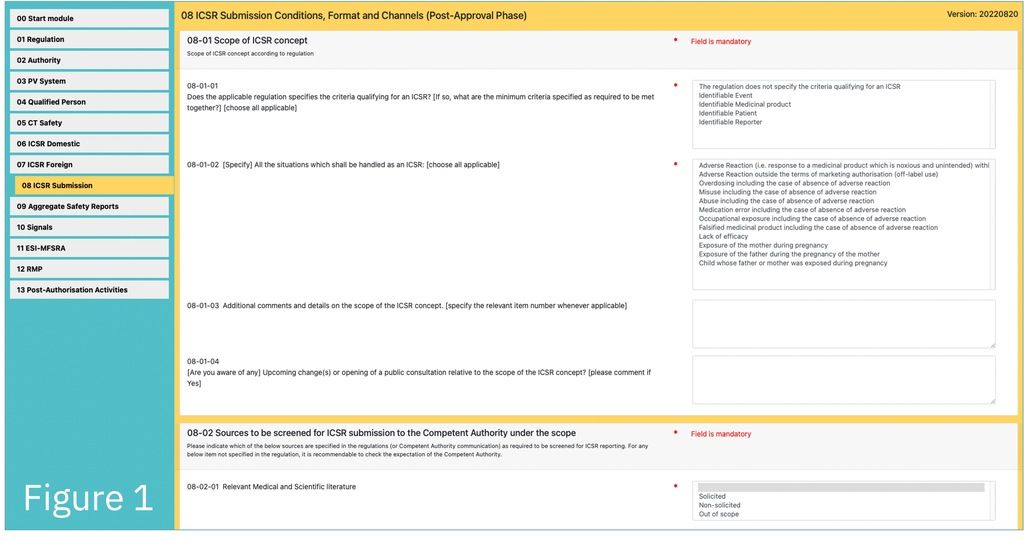
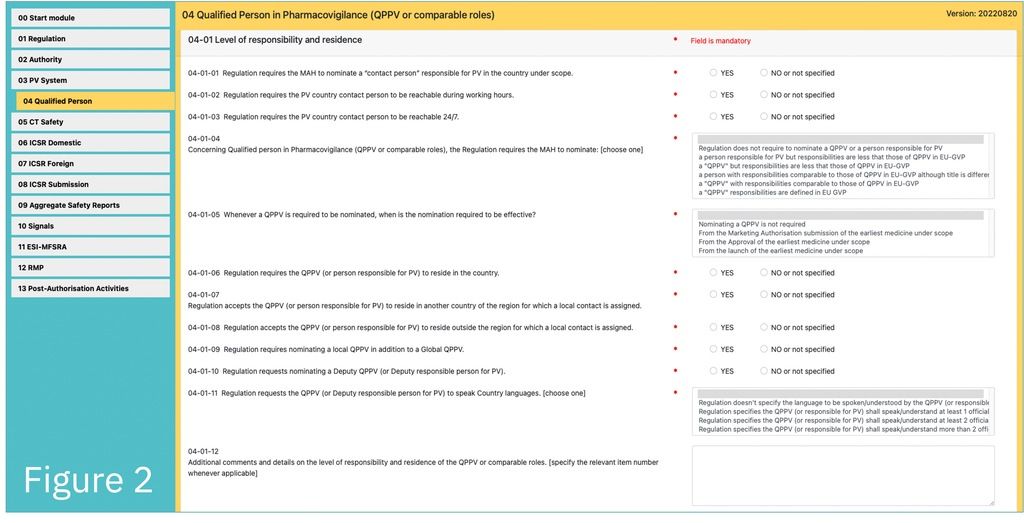

PV Requirement Country Profiles are produced from the information collected with the PVReg-Profiler. This information is to be used to customise the GPPC for the regulatory system under scope.
RESULTS
During a pilot phase, pharmacovigilance professionals experienced complying with the Country regulation under scope, were appointed for filling the PVReg-Profiler (Method A). This approach revealed diverging interpretations across appointed Experts. This led to consider alternative approaches and to offer the relevant NRA the opportunity to select the preferred method. (Methods B, C, D). Among them, Method C offers the relevant NRA the opportunity to receive details on how the applicable regulation is actually understood by the stakeholders committed to apply it. Accordingly, whatever the method applied, the relevant NRA will be approached seeking for their endorsement of the content of the PV Requirement Country Profile for their Country.
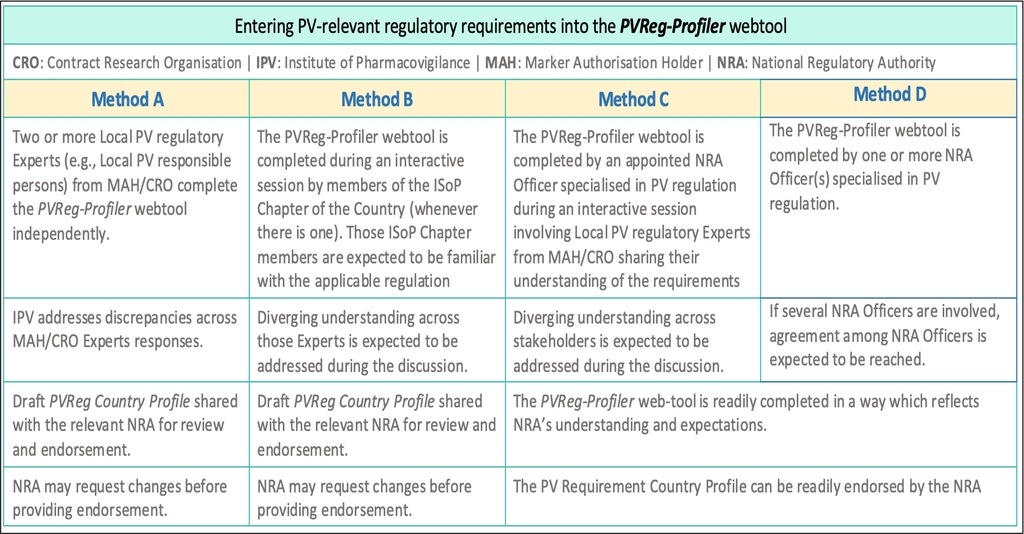
DISCUSSION
Whilst the primary purpose of the PVReg-Profiler is to collect the information necessary for customising the GPPC for specific countries, IPV’s PVReg initiative is believed to be of interest for multiple stakeholders including NRAs, academic institutions undertaking regulatory research, market authorisation holders, contract research organisation, as well as public health organisations involved in regulatory convergence, strengthening and reliance in the field of pharmacovigilance (Ref. 1, 2 and 3). IPV is interested in developing specific analytic tools in collaborations with those stakeholders.
CONCLUSION
IPV welcomes partnering with NRAs for creating PV Requirement Country Profile, and collaborating with stakeholders interested in using the information collected by the PVReg System.
REFERENCES
1. WHO Global Benchmarking Tool (GBT) for Evaluation of National Regulatory System of Medical Products - Revision VI. World Health Organisation, 2017.
2. Manual for benchmarking of the national regulatory system of medical products and formulation of institutional development plans, WHO, 2021.
3. Policy on the evaluation and designation of regulatory authorities as WHO listed authorities, World Health Organisation, 2021.
PVReg-Repository
A comprehensive global repository of pharmacovigilance-relevant regulations
BACKGROUND
The context of rapid and diversified regulatory strengthening (Ref.1), makes necessary to establish a comprehensive global repository of pharmacovigilance-relevant regulations including laws, ordinances, good vigilance practice document and guidelines. Surprisingly no such repository had been developed before the launch the PVRegulations initiative in 2021. Subsequently, evident synergies led to incorporating this repository into the PVReg knowledge management system developed by the Institute of Pharmacovigilance (IPV), to substantiate the Global Pharmacovigilance Certification programme (GPPC).
METHODS
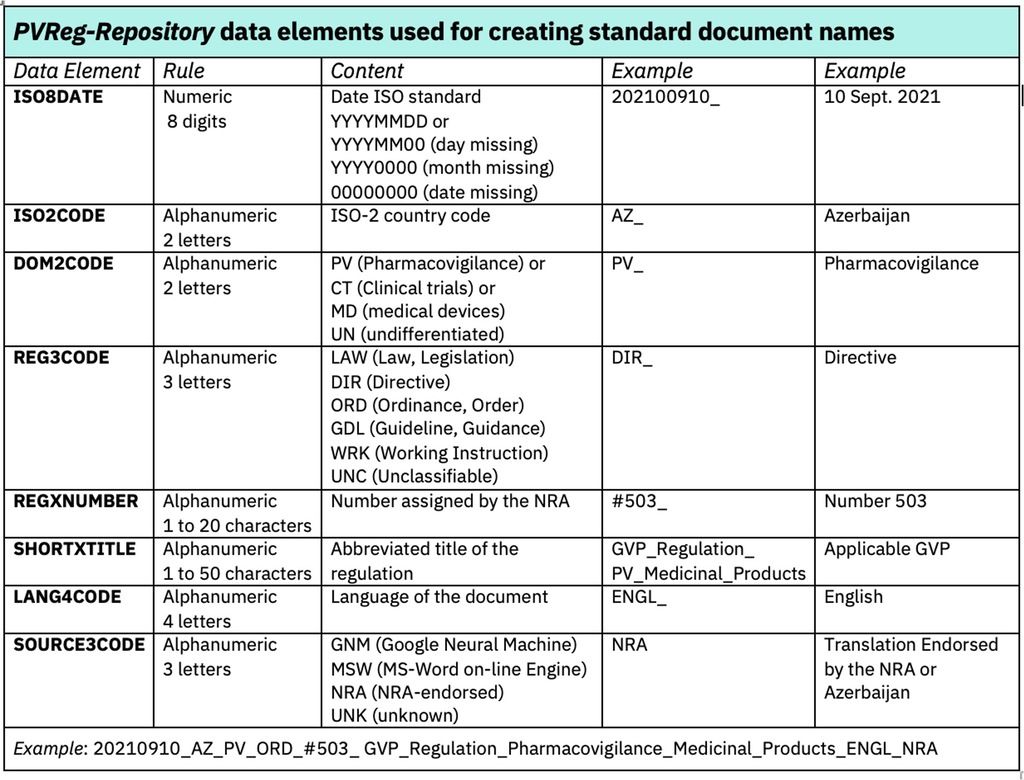
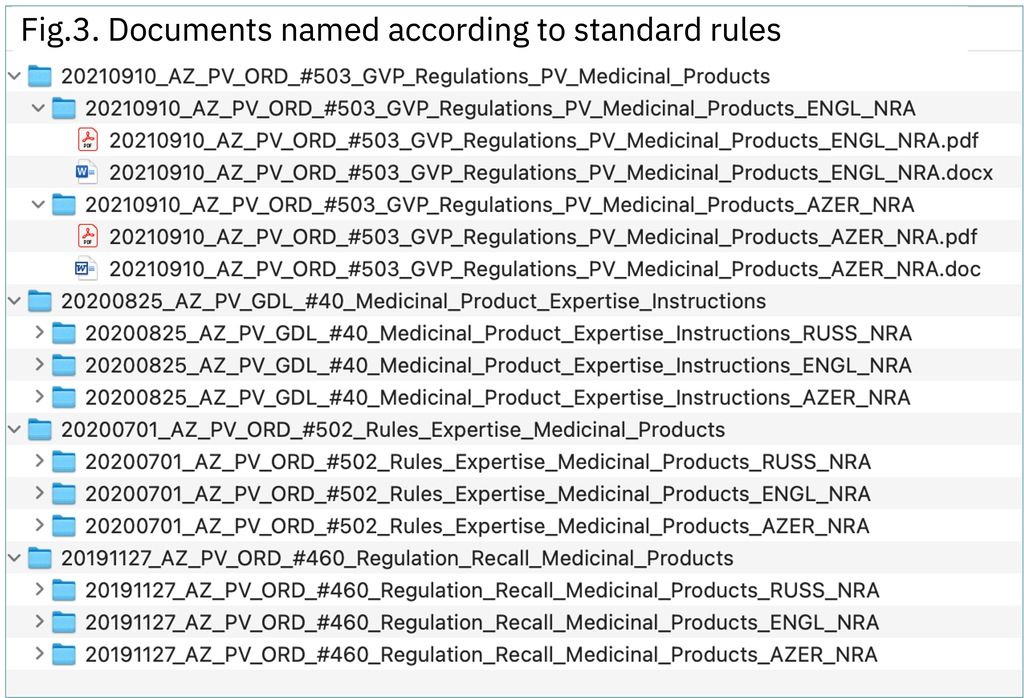
The PVReg-Repository is designed according to a simple and consistent easy to navigate architecture aimed at providing access to targeted documents with a few clicks without need for specific training. Standard document naming rules were established in order to readily provide basic information on the document. Those document name components will be used as metadata when, in a second step, the PVReg-Repository will be upgraded to a document management system to enable more powerful document searching.
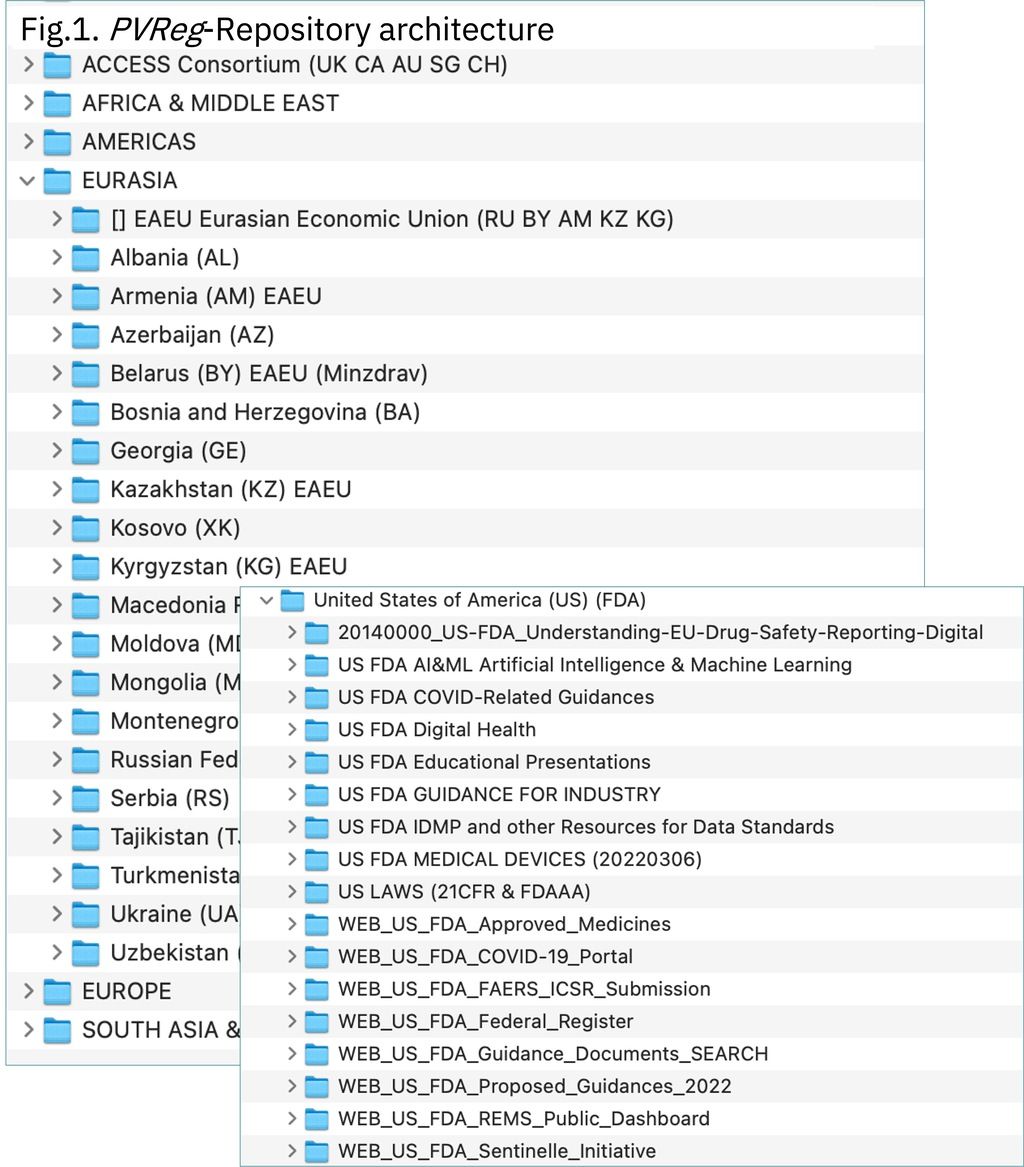
RESULTS
The PVReg-Repository is primarily focused on PV-relevant regulations. However, its scope is being extended to regulations applicable to clinical trials and Medical Devices for which specific folders are being created. To capture regulatory contents available on html format only, URLs had to be added (Fig.1). The main challenge faced when filling the repository was the diversity of document formats and languages to be addressed ranging from keyword-searchable PDF or Microsoft-word documents in English to unsearchable PDFs resulting from scanning a poorly readable telefax in national language. In a few cases, PDFs generated from MS-Word documents were content-locked resulting in making their content unsearchable and preventing running machine-operated translation from National language to English. In such cases, the use of Optical Character Recognition (OCR) was tested but very resource-consuming. Both Google Neural Machine Translation (GNMT) and the on-line Microsoft full-document translation Engine available in MS-Word 365 were tested. In most of the languages tested, both systems provided workable translations however not reaching the quality of a document which may be readily endorsed by the relevant National Regulatory Authority.
DISCUSSION
Early experience from filling and maintaining the PVReg-Repository demonstrated the value of a collaborative networking with National Regulatory Authorities (NRAs) in order a) to ensure the timely awareness of newly released regulations and the completeness of country folders, b) to confirm the regulatory categorisation (REG3CODE, Fig.2.b), c) to receive documents in the most suitable format to run machine translation, d) and to improve translations aiming at making validated versions available. In addition to constituting a source of knowledge substantiating the GPPC, the PVReg-Repository appears to be of interest for public health and Industry stakeholders, as well as for academic institutions involved in regulatory science and research.
CONCLUSION
Combined with the PVReg-Profiling system the PVReg-Repository is expected become an essential resource for monitoring the change of the safety regulatory environment at the country, regional and global level.
REFERENCES
1. WHO Global Benchmarking Tool (GBT) for Evaluation of National Regulatory System of Medical Products - Revision VI. World Health Organisation, 2017.


